Validation Case Study: Blood and Plasma
About Client
Our client operates regional plasma donation centers. Plasma is used to create medicine that treats chronic illnesses, including immune disorders, liver disease, bleeding disorders, and cancer. There simply is not enough plasma for everyone who needs it, and our client aims to change that - one donation at a time.
Business Challenge
Our client expanded their network with seven new blood plasma donation centers to meet the increasing demand for plasma collection and storage. Each site required plasma freezers capable of maintaining stringent temperature requirements to ensure product integrity and compliance with regulatory standards. To prepare these sites for inspection readiness, all units had to be fully qualified prior to operational use.
Project Challenges
TRANSCAT’s Validation team was tasked with completing the Installation, Operational, and Performance Qualification (IOPQ) of multiple large-capacity plasma freezers across seven centers. The work needed to be standardized yet executed under site-specific conditions, while ensuring compliance with regulatory guidelines and meeting aggressive timelines for center openings.
Scope of Work
TRANSCAT’s onsite engineers and support staff executed full qualification protocols for the Plasma Freezers, including:
- Installation Qualification (IQ):
Verified freezer installation, mechanical integrity, environmental conditions, equipment identification, and calibration/preventive maintenance schedules.
- Operational Qualification (OQ):
Confirmed that control systems, alarms, temperature settings, and monitoring components functioned in accordance with manufacturer and protocol requirements.
- Performance Qualification (PQ):
Conducted comprehensive freezer performance testing, including:
- 12-hour empty chamber mapping at -40°C
- 24-hour loaded chamber mapping at -40°C
- Daily packing and bi-weekly shipping movement studies to simulate operational use
- Core freeze study ensuring plasma samples cooled to ≤ -25°C within 12 hours
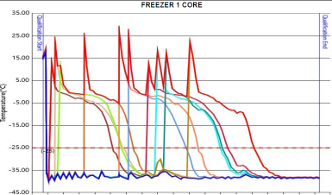
- Thermocouple calibration and verification for data integrity


